Structure-

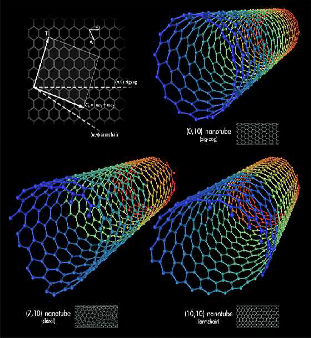
♤ The bonding in carbon nanotubes is sp², with each atom joined to three neighbours, as in graphite. The tubes can therefore be considered as rolled-up graphene sheets (graphene is an individual graphite layer). There are three distinct ways in which a graphene sheet can be rolled into a tube.
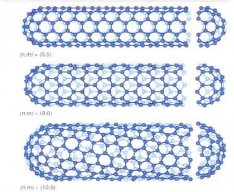

♤ Carbon nanotubes are long chains of carbon held together by the strongest bond in all chemistry, the sacred sp2 bond, even stronger than the sp3 bonds that hold together diamond. Carbon nanotubes have numerous remarkable physical properties, including ballistic electron transport (making them ideal for electronics) and so much tensile strength that they are the only substance that could be used to build a space elevator. The specific strength of carbon nanotubes is 48,000 kN·m/kg, the best of known materials, compared to high-carbon steel’s 154 kN·/kg. That’s 300 times stronger than steel. You could build towers hundreds of kilometers high with it.
♤ Carbon Nanotube Technology can be used for a wide range of new and existing applications:
Conductive plastics
Structural composite materials
Flat-panel displays
Gas storage
Antifouling paint
Micro- and nano-electronics
Radar-absorbing coating
Technical textiles
Ultra-capacitors
Atomic Force Microscope (AFM) tips
Batteries with improved lifetime
Biosensors for harmful gases
Extra strong fibers