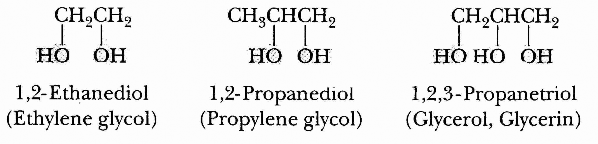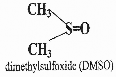36. CRYOPROTECTANT-
♤ A cryoprotectant is a substance that is used to protect biological tissue from freezing damage (i.e. that due to ice formation). Arctic and Antarctic insects, fish and amphibians create cryoprotectants (antifreeze compounds and antifreeze proteins) in their bodies to minimize freezing damage during cold winter periods. Insects most often use sugars or polyols as cryoprotectants. Arctic frogs use glucose, but Arctic salamanders create glycerol in their livers for use as a cryoprotectant. Cryoprotectants operate simply by increasing the solute concentration in cells. However, in order to be biologically viable they must (1) easily penetrate cells, and (2) not be toxic to the cell.
♤ Conventional cryoprotectants are glycols (alcohols containing at least two hydroxyl groups), such as ethylene glycol[citation needed], propylene glycol, and glycerol. Ethylene glycol is commonly used as automobile antifreeze, and propylene glycol has been used to reduce ice formation in ice cream. Dimethyl sulfoxide (DMSO) is also regarded as a conventional cryoprotectant. Glycerol and DMSO have been used for decades by cryobiologists to reduce ice formation in sperm and embryos that are cold- preserved in liquid nitrogen.