18.1.2. Influence of other factors
Various factors can locally influence the chemical reactions of CO2 with sea water and add to the effects to ocean acidification. For example,
i. Acid rain
Acid rain can have a pH between 1 and 6 and has impact on surface ocean chemistry. It has major effect on ocean acidification locally and regionally but very small globally.
ii. Eutrophication
Coastal waters are also affected by excess nutrient inputs, mostly nitrogen, from agriculture, fertilizers and sewage. The resulting eutrophication leads to large plankton blooms, and when these blooms collapse and sink to the sea bed the subsequent respiration of bacteria decomposing the algae leads to a decrease in sea water oxygen and an increase in CO2 (a decline in pH).
How it reacts?
The term ‘ocean acidification’ summarizes several processes that occur when CO2 reacts with sea water.
Two reactions are particularly important. Firstly, the formation of carbonic acid with subsequent release of hydrogen ions:
CO2 + H2O
(Carbon dioxide) + (Water) H2CO3 H+ HCO3--
(Carbonic acid) (Hydrogen ions) + (Bicarbonate ions)
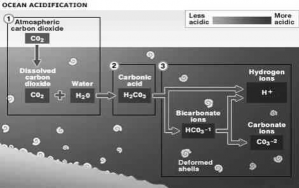
The above reaction and release of hydrogen ions increases acidity and thus pH level is reduced. A second reaction, between carbonate ions, CO2 and water produces bicarbonate ions.
The combined effect of both these reactions not only increases acidity but also lowers the availability of
carbonate ions.
